Chinese and US drug watchdogs have reached a consensus to be part of each other's inspection and investigation against counterfeits and substandard drugs, the State Food and Drug Administration (SFDA) said yesterday.
The first-ever Sino-US agreement on drug and medical equipment safety signed on Tuesday led to the consensus, marking a substantial step forward for the two countries in better supervision on drugs, SFDA spokesperson Yan Jiangying said.
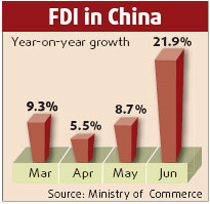
Considering the volume of drug trade between China and the US, a partnership that ensures product safety and covers all links of import and export is very important, Yan said.
The agreement addresses drugs and medical equipment traded between China and the US such as medicines used to treat impotency, dietary supplements, glucose test strips and condoms from China; and US-made insulin, heart pacemakers and diagnostic kits used for HIV and hepatitis tests, she said.
The US will have to provide China with information on the listed products, recalls, warning letters and adverse side effects.
And Chinese drug and medical equipment manufacturers on the US list have to register with the SFDA. The registration will help track and give the number of companies exporting to the US, and help investigators if and when a problem occurs to track them down, Yan said.
For product safety, the US Food and Drug Administration (FDA) is entitled to launch a joint probe with its Chinese counterpart, the SFDA, against a registered firm if its products are found unsafe.
The items listed by both sides are just a sliver of health-related products traded between China and the US, she said. The list will be expanded in the future.
The two countries will set up a special working group that will meet within 120 days to devise a plan to implement the agreement, and set performance measurements to evaluate progress in enhancing drug and medical equipment safety, Yan said.
Also, a mutual crisis alert mechanism has been initiated under the agreement on food and feed safety the two sides signed on Tuesday, according to the General Administration of Quality Supervision, Inspection and Quarantine.
Under the mechanism, each side has to notify the other of any unsafe food products within 48 hours of its discovery or recall. The other country, in turn, has to give feedbacks.
(China Daily December 13, 2007)