Hefei firm sees key breakthrough in medical equipment
Updated: 2023-03-28
|chinadaily.com.cn
The MOBINEURO Alita 1.5T Intraoperative Magnetic Resonance System developed by Sino Canada Health Engineering Research Institute (Hefei) Co Ltd, or SCHERI, passed the US Food and Drug Administration's (FDA) audit on March 21, making it the first product of its kind to obtain FDA marketing approval in China.
SCHERI is a resident company in Hefei National High-tech Industry Development Zone, located in Hefei city, provincial capital of East China's Anhui.
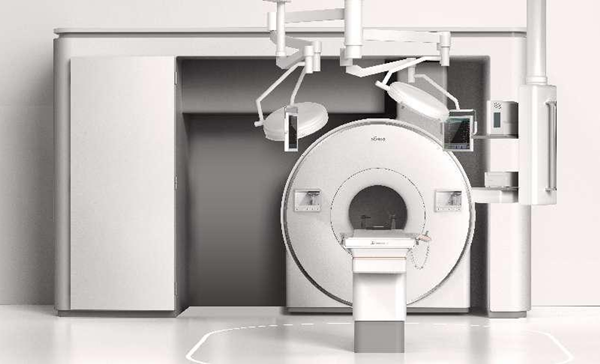
A view of the MOBINEURO Alita 1.5T Intraoperative Magnetic Resonance System developed by Hefei high-tech zone-based SCHERI. [Photo/WeChat ID: hefeigaoxinfabu]
The system has completely independent intellectual property rights, breaking the decades-long product monopoly of international manufacturers in this field and realizing the domestic replacement of large medical imaging equipment for the brain. The product is considered to hold great value in terms of neurosurgery clinical applications, multimodal precision medicine and brain-machine interface research.
MOBINEURO Alita 1.5T is supported by a series of local science and technology policies from the Institute of Artificial Intelligence at the Hefei Comprehensive National Science Center. It was developed eight months ahead of schedule, achieving a major breakthrough in the R&D, manufacturing and commercialization of high-end medical imaging equipment in Anhui province.
Hefei high-tech zone has invested nearly 30 billion yuan ($4.36 billion) to develop the Institute of Artificial Intelligence. Some 34 of the center's achievements have gained national recognition and the facility is now accelerating the commercialization of over 200 cutting-edge technologies.
