WHO authorizes Sinovac's COVID-19 vaccine for emergency use
- By Zhang Rui
 0 Comment(s)
0 Comment(s) Print
Print E-mail China.org.cn, June 2, 2021
E-mail China.org.cn, June 2, 2021
The CoronaVac vaccine developed by leading Chinese pharmaceutical company Sinovac has now been approved for emergency use by the World Health Organization (WHO), while its use in the mass vaccination of a small Brazilian town shows promising prospects.
CoronaVac is an inactivated coronavirus vaccine developed by Sinovac Life Sciences Co., Ltd., a subsidiary of Sinovac Biotech Ltd. In June 2020, it became the first vaccine approved for emergency use in China and was further granted conditional marketing authorization on Feb. 5, 2021.
"I am happy to announce that the Sinovac coronavirus vaccine has been given WHO Emergency Use Listing after being found to be safe, effective, and quality assured following two doses of the vaccine," WHO Director-General Tedros Adhanom Ghebreyesus announced at a WHO press briefing on Tuesday. "Furthermore, the easy storage requirements of CoronaVac make it very suitable for low resource settings."
Yin Weidong, the chairman and CEO of Sinovac, said that phase 3 trials and follow-up real-world studies in Brazil, Turkey, Indonesia and Chile represent good examples of the collaborative, global action against the pandemic. These studies have provided a solid scientific foundation for CoronaVac to be approved by more than 40 countries, as well as the WHO.
"These achievements couldn't have happened without the efforts of global partners and scientists. Sinovac will continue to participate in pandemic prevention and control actions, acknowledging the value of China's COVID-19 vaccines as a global public good and contributing to the international victory over COVID-19," he said.
Sinovac had submitted data from clinical and non-clinical research, as well as manufacturing and control, to the WHO review group for evaluation. This February, the WHO also carried out an on-site inspection of Sinovac's vaccine manufacturing facilities, and examined its quality management system.
The Strategic Advisory Group of Experts on Immunization (SAGE) also conducted a systematic review of CoronaVac. It showed that the Sinovac vaccine had been authorized by 32 countries or jurisdictions for use in adults 18 years or older, with 260 million doses having been distributed to the public in China and overseas. No safety concerns have been identified from pre-clinical or reproductive toxicology studies, while most adverse events were mild to moderate. Therefore, it concluded that the benefits of Sinovac's vaccine are greater than the known risks.
According to SAGE, it recommended two doses of CoronaVac for adults aged 18 and above with an interval of two to four weeks. The efficacy results showed that the vaccine prevented severe COVID-19 and hospitalization in 100% of those studied.
CoronaVac is the second WHO-approved vaccine from China after the Sinopharm vaccine, which was also cleared last month for emergency use. The two Chinese vaccines are expected to accelerate vaccine rollouts in many low- and middle-income countries through the WHO's COVAX initiative.
A day before the WHO announcement, there was a good news from a unique experiment in Brazil: In the small town of Serrana in the countryside of Sao Paulo state, CoronaVac was administered to 27,160 residents, protecting both vaccinated and non-vaccinated. This created a collective "immunological belt," drastically reducing transmission of the virus, causing a huge reduction of symptomatic cases by 80% and deaths by 95% after the second vaccination of the last group.
"We have achieved exemplary large-scale epidemiological laboratory work to demonstrate how the effectiveness of the vaccine can save lives and control the pandemic," said Dimas Covas, president of the Butantan Institute which produces CoronaVac locally, at a press conference held by the state government on Monday.
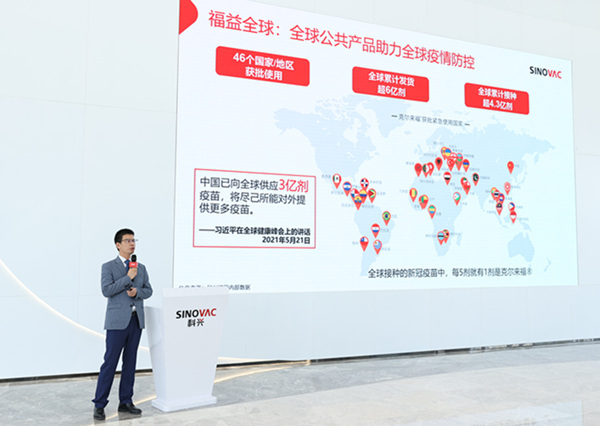
As of May 31, Sinovac has provided CoronaVac to nearly 40 countries and regions, including China's domestic market. The total supply has already exceeded 600 million doses in 300 days since the vaccine was initiated on Jan. 28, according to Liu Peicheng, a spokesperson for Sinovac at a press conference on Tuesday.
"The safety and effectiveness of CoronaVac has been verified all over the world," Liu said.
Sinovac also launched the BRICS Vaccine R&D Center — China Center on May 28, combining online and offline methods to promote joint research, vaccine development and testing, and factory building, as well as promoting authorized production and mutual recognition of standards between BRICS nations.
Sinovac will provide funding to the center to carry out collaborations in disease and pathogen surveillance, basic research, clinical studies, real-world studies, vaccine production, and studies to support decision-making on immunization strategy. It will be expanded to the "BRICS+" concept, in order to support and encourage comprehensive partnership among BRICS countries as well as with governments, universities, research institutions, and industries worldwide.






Go to Forum >>0 Comment(s)