More tainted capsules, companies found
 0 Comment(s)
0 Comment(s) Print
Print E-mail Shanghai Daily, April 28, 2012
E-mail Shanghai Daily, April 28, 2012
In the latest nationwide inspections of medicine capsules, 74 batches made by 15 pharmaceutical companies were found to contain excessive chromium, the State Food and Drug Administration said yesterday.
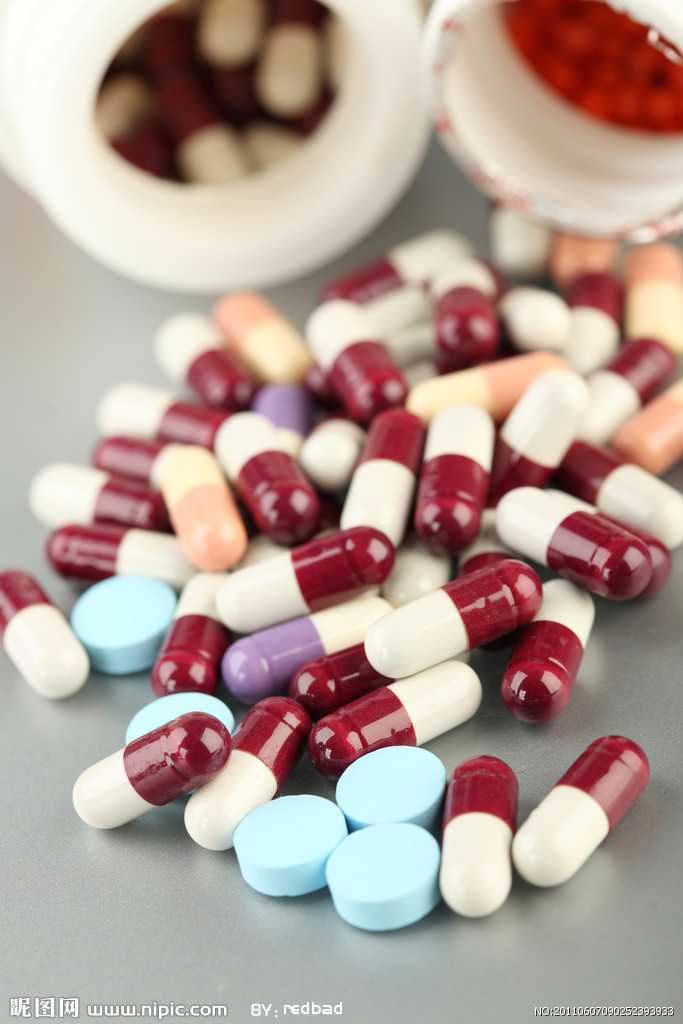 |
The administration said it conducted checks on 117 producers and 941 batches of capsules. The toxic capsules will be recalled and destroyed, officials said. They said nine of the 15 companies were in Zhejiang Province, the main production area, while the others were in Chongqing and the provinces of Henan and Sichuan.
Producers' licenses will be suspended and people directly responsible will be "removed out of the medicine production industry," officials said.
The scandal came to light when China Central Television reported that several companies were using industrial gelatin to make drug capsules. The gelatin contained chromium, which can cause kidney and liver damage and lead to cancer.
"Daily management and supervision is severely lax in these companies," SFDA officials said. They have ordered all capsule manufacturers to list their raw materials on the local SFDA website so the public can be made aware of the ingredients.
The SFDA issued an emergency notice calling for a halt to the sale and consumption of a list of drugs that may have been packed into tainted capsules. Many people started avoiding all drug capsules after the scandal was exposed.
Along with the checks, authorities have begun investigations into possible dereliction of duty among law enforcement and safety supervisors.






Go to Forum >>0 Comment(s)